Carnot's Heat Engine Employs Which of the Following
Isentropic compression of the gas. For example when the hot reservoir have T hot of 400C 673K and T cold of about 20C 293K the maximum ideal efficiency will be.

Carnot Heat Pump Or Carnot Refrigerator
Carnots heat engine employs.

. Isentropic reversible adiabatic expansion of the gas. D two adiabatic processes and two isobaric processes. Advanced Physics questions and answers.
A heat engine that works according to the Carnot cycle is known as a Carnot engine. Reversible or changeable isothermal compression of the gas at the cold temperature. The efficiency of a Carnot heat engine is given by the Formula.
The Carnot engine cycle when acting as a heat engine consists of the following steps. Carnot efficiency depends on high temperature and low temperatures between which the heat engine operates. Show transcribed image text Expert Answer.
Isentropic compression of the gas. The cycle of this engine is called the Carnot cycle. Efficiency of Carnot engine e Solution.
Let V 1 P 1 be the initial volume and pressure of the gas respectively. Any amount of heat can be extracted from it without changing its temperature. Find step-by-step Physics solutions and your answer to the following textbook question.
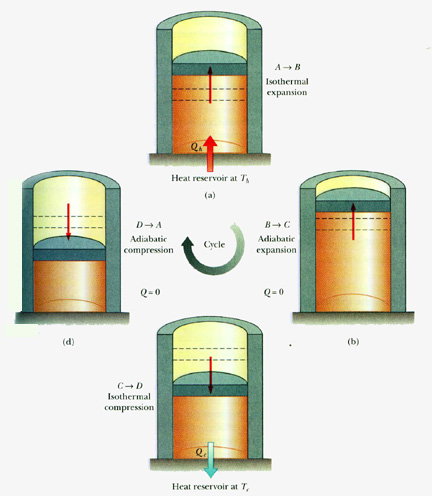
Let us consider one mole of an ideal gas enclosed in the cylinder. Reversible or changeable adiabatic Isentropic expansion of the gas. The Carnot cycle when acting as a heat engine consists of the following four steps.
A Carnot engine employs 15 mol of nitrogen gas as a working substance which is considered as an ideal diatomic gas with gamma 75 at the working temperatures of the engine. It is not an actual thermodynamic. A Carnot heat engine receives heat from a reservoir at 900 C at a rate of 800 kJmin and rejects the waste heat to the ambient air at 27 C.
W 13600 200 Joule. Ef f C 1 T c T h E f. T h o t 540 o C 273 813 K T c o l d 20 o C 273 293.
Who are the experts. Camots heat engine employs. E two isothermal processes and two isobaric processes.
Experts are tested by Chegg as specialists in their subject area. High temperature T H 500 K. If Q 2 0 then efficiency 100.
1 T cold T hot 1. The Carnot engine cycle when behaving as a heat engine contains the following steps. The Carnot engine has the following four stages of operations.
It is the source of heat maintained at constant high temperature TH. Michael Fowler The Ultimate in Fuel Efficiency All standard heat engines steam gasoline diesel work by supplying heat to a gas the gas then expands in a cylinder and pushes a piston to do its work. The efficiencies of all reversible engines Carnot heat engines operating between the same constant temperature reservoirs are the same regardless of the working substance employed or the operation details.
We are given both temperatures. This is known as the Carnot engine. Thus the efficiency will completely depend on the temperatures of the source and the sink and is not a constant However for a given set of source temperature and sink temperature the Carnot heat engine is the most efficient engine possible.
During this step the fuel is burned creating the hot temperature and causing the working fluid or gas to expand. Reversible isothermal expansion of the gas at the hot temperature. E 500 350 500.
Carnot engine is a reversible heat engine which works on Carnot cycle. Calculate the Carnot efficiency of the power plant. In 1824 Nicolas Leonard Sadi Carnot invented the Carnot engine.
Entropy is produced due to the finite time heat exchange between the endoreversible engine the rectangle region in Fig. Then Efficiency η W Q 1 Q 1-Q 2 Q 1 1-Q 2 Q 1 which is the efficiency of the heat engine. Efficiency of Carnot engine.
That is Qc Qh T c T h Q c Q h T c T h for a Carnot engine so that the maximum or Carnot efficiency EffC is given by. It is a cold body maintained at a constant low temperature TL. 61 and the thermal reservoirs.
W e Q 1. For which combination of working temperatures of source and sink the efficiency of Carnots heat engine is maximum. We know that Work done W Q 1-Q 2.
It can absorb any amount of heat. According to the Carnot principle no engine can be more efficient than a reversible engine a Carnot heat engine operating between the same high temperature and low temperature reservoirs. Work done by Carnot engine.
What Carnot found was that for a perfect heat engine the ratio Qc Qh Q c Q h equals the ratio of the absolute temperatures of the heat reservoirs. View solution Efficiency of Carnot engine is 1 0 0 if. However the temperatures need to be converted to Kelvin.
A system undergoing a Carnot cycle is called a Carnot heat engine. Based on the graph below what is the efficiency of the Carnot engine. It is an ideal case where the efficiency is 100.
Carnot cycle comprises of four processes. View solution View more. Heat input Q 1.
The catch is that the. Consider a reversible heat. It is a Carnot engine which experiences external irreversibility due to finite heat exchange between the engine and the hot and cold thermal reservoirs.
1 T2T1 which has been derived above. The efficiency of the Carnot engine is given as η Work done Heat input. We review their content and use your feedback to keep the quality high.
A two adiabatic processes and two isothermal processes B two adiabatic processes and two isochoric processes C two isothermal processes and two isochoric processes. Reversible isothermal compression of the gas at the cold temperature. View solution The efficiency of a carnot engine working between 800 K and 500 K is.
Low temperature T L 350 K. This simplest heat engine is called the Carnot engine for which one complete heatingcooling expandingcontracting cycle back to the original gas volume and temperature is a Carnot cycle named after Sadi Carnot who in 1820 derived the correct formula for the maximum possible efficiency of such a heat engine in terms of the maximum and minimum gas temperatures. E T H T L T H.
The Carnot Cycle Applet here. The Carnot cycle provides an estimation of the extreme possible efficiency that a heat engine converts heat into output work on the contrary working between two reservoirs hot and cold. The Carnot cycle goes in the cycle ABCDA with AB being an isothermal expansion.
Reversible and isothermal expansion of the working fluid at the hot temperature T H isothermal heat addition. The carnot engine has four parts which are given below. Carnot Heat engine based on Carnot Cycle was a concept developed by Nicolas Leonard Sadi Carnot 1796-1832 a French Military Engineer and Physicist so that one can visualize a reversible heat engine in practice.

Carnot Cycle An Overview Sciencedirect Topics

Carnot Cycle And Reversed Carnot Cycle Electrical4u

Solved The Three Carnot Engines Shown In The Drawing Operate With Hot And Col Solutioninn

Carnot S Perfect Heat Engine The Second Law Of Thermodynamics Restated College Physics I Openstax Cnx

Carnot S Perfect Heat Engine The Second Law Of Thermodynamics Restated Physics

Carnot Engine Cycle Principles Theorem Efficiency With Videos

What Is A Carnot Engine How Does A Carnot Cycle Work

Heat Engine Definition Heat Engine Efficiency Carnot Engine

Heat Engine Definition Heat Engine Efficiency Carnot Engine
Steam Engine Physics Carnot Cycle

Carnot S Theorem With Explanation And Its Applications

Carnot Cycle Equation Efficiency Engine Video Lesson Transcript Study Com

What Is A Carnot Engine How Does A Carnot Cycle Work

Carnot Engine Carnot Theorem Carnot Cycle Working Efficiency

Sketch Of The Car Composed Of Internal Carnot Heat Engine And Carnot Download Scientific Diagram

Comments
Post a Comment